Sol-Gel Chemistry
Hydrolysis and Condensation
Reactions of Alkoxysilanes
The sol-gel technique is an important preparation method for polymeric inorganic materials. It allows for the preparation of glassy materials of high-purity at temperatures far below their melting point and can be used to produce a large number of materials in different shapes and forms including coatings, films, fibers as well as bulk parts which are difficult to obtain by conventional processes due to their high melting temperature or tendency to crystallize.
The process generally starts with metal alkoxides, which undergo a series of hydrolysis and condensation reactions to give sols and gels. The most important class of alkoxides are alkoxysilanes, such as tetramethoxysilane (TMOS) and tetraethoxysilane (TEOS). Other alkoxides commonly used in a sol-gel process include aluminates, titanates, and zirconates which, however, are used on much smaller scale and often in combination with other materials such as TEOS. In the case of alkoxysilanes, the chemical reactions during sol-gel conversion can be formally described by the following three reactions:
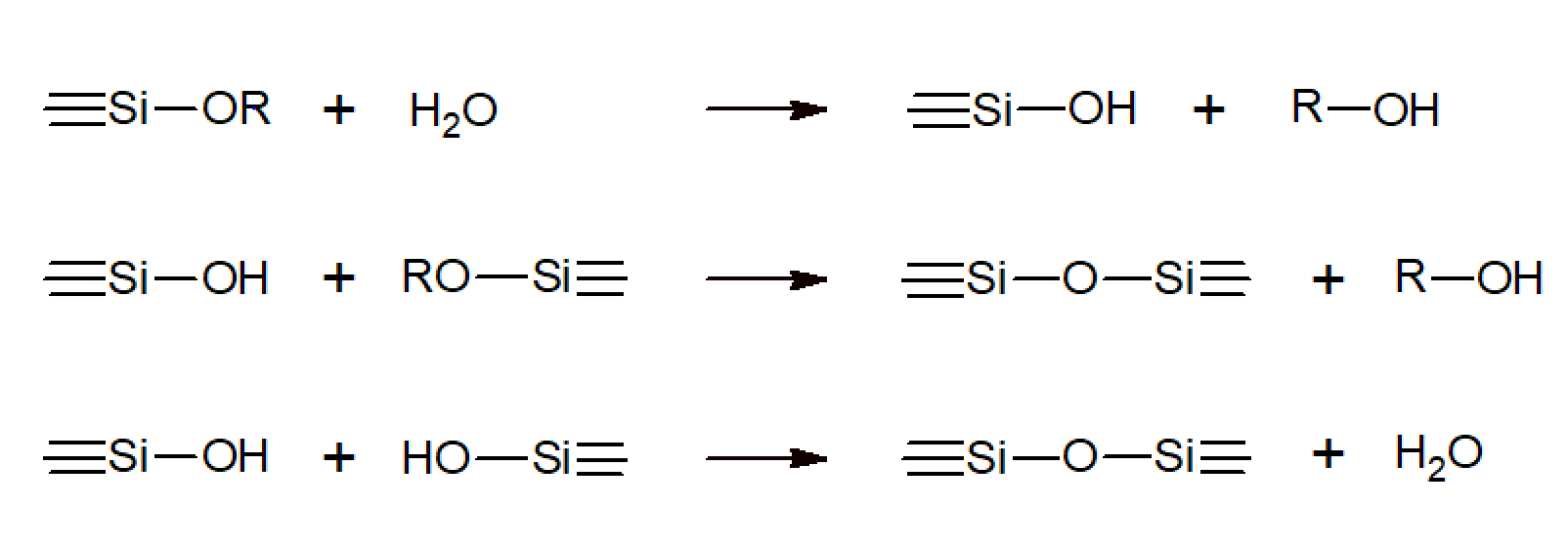
The hydrolysis of alkoxy groups (Si-OR) always precedes the condensation reactions and generates silanol groups (Si-OH) which then condense to Si-O-Si bonds by either alcohol or (more common) water elimination. The two step mechanism of alkoxy polymerizations is an important difference to typical organic polymerization reactions.
The alkoxysilanes are usually not soluble in the (released) water. Therefore, a common solvent such as a low MW alcohol is often added as a homogenizing agent. However, the addition of an alcohol is not always neccessary since alcohol is produced as a by-product during hydrolysis of the alkoxy groups which is sometimes sufficient to homogenize the mixture. The hydrolysis is typically initiated by addition of a small amount of water under acidic, neutral, or basic conditions. It has been postulated1 that the reaction follows a second-order mechanism and can be described as a bimolecular nucleophilic substitution (Sn2-type) forming silanol groups.1-3 For example, under strongly alkaline conditions, the hydrolysis proceeds by nucelophilic attack of a hydroxide ion (-OH) at the silicon atom with an alkoxy group (RO-) as the leaving group:1
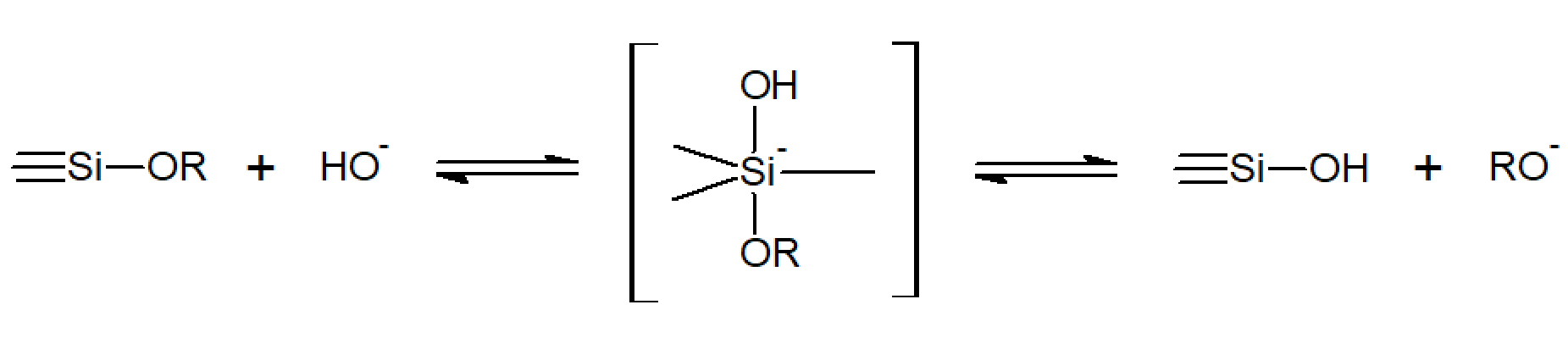
The inductive effect of the substituents attached to the silicon atom strongly affects the reaction rate, that is, they either decrease or increase the electron density at the silicon atom, and thus stabilize or destabilize the transition state during hydrolysis. For base catalyzed hydrolysis, the electron density at the silicon atom should be lowered because the negative charge of the transition state is then stabilized. Therefore, the reaction rates for hydrolysis and condensation under alkaline conditions increases with the number and electronegativity of the electron-withdrawing groups. For acidic conditions, a positively charged transition state has to be stabilized and, therefore, the reaction rate increases in the same order as the electron density at silicon atom. Since the electron density at the silicon atom decreases in the order ≡Si-R > ≡Si-OR > ≡Si-OH > ≡Si-O-Si≡, we conclude that under acidic conditions alkylalkoxysilanes Si(OR)4-x(R)x hydrolyze faster than alkoxysilanes, Si(OR)4 which, in turn, hydrolyze faster than partially hydrolyzed siloxanes Si(OR)4-x(OH)x and condensed oligomeric siloxanes which have additional Si-O-Si bonds, and vice versa under alkaline conditions. We also conclude that more branched structures are obtained under basic conditions than under acidic conditions, because reactions at silicon atoms with fewer Si-O-Si bonds are favored under acidic conditions as well as reactions at terminal silicon atoms.
The rate of hydrolysis also depends on the size of the alkoxy and alkyl substituents. With increasing size of the substituents, steric crowding around the silicon atom incresases which, in turn, reduces the reactivity of the alkoxysilane. For this reason, tertraethoxysilane (TEOS) hydrolyzes slower than tetramethoxysilane (TMOS). The same is true for organyl alkoxysilanes with large alkyl groups or phenyl rings.
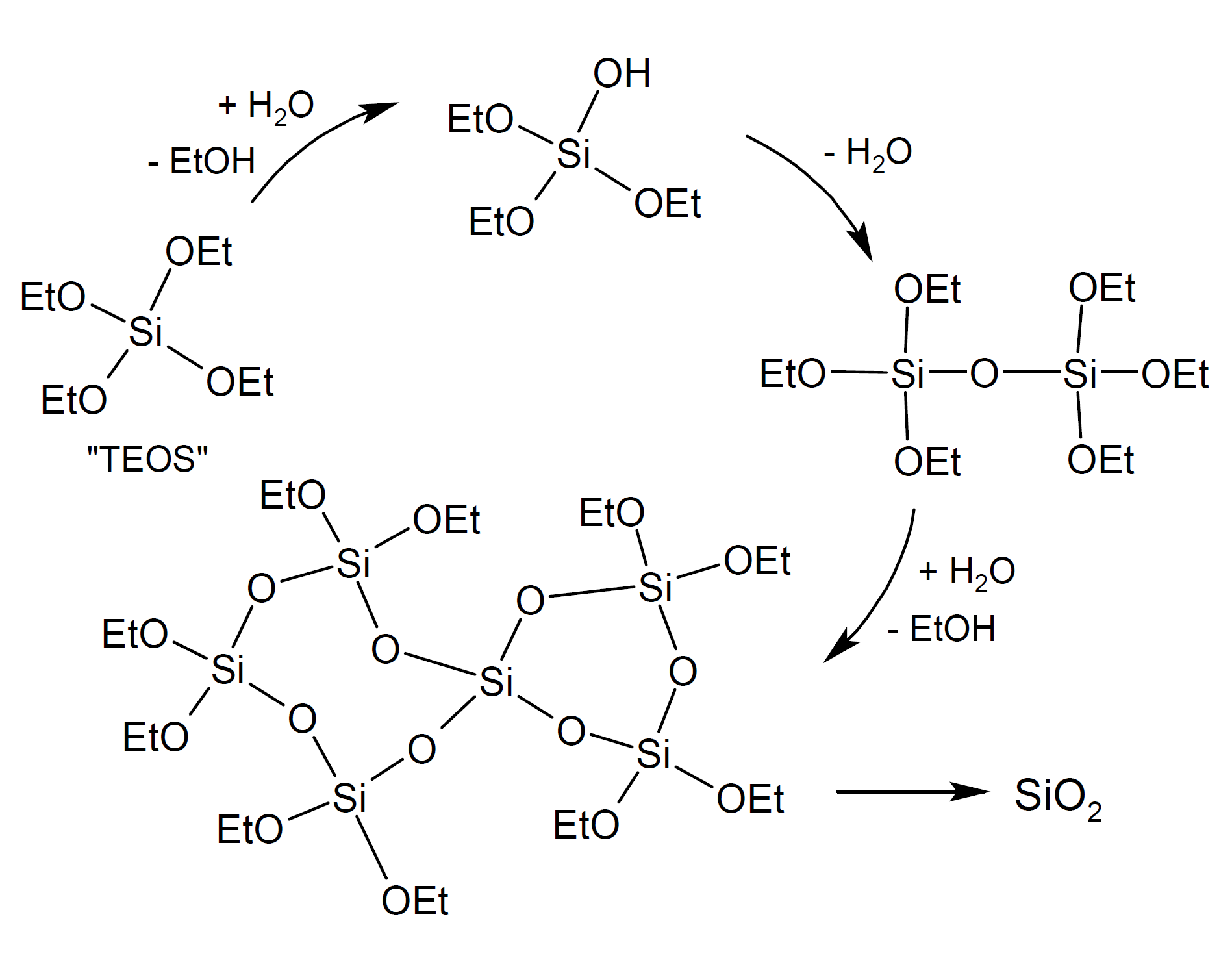
The polymerization of alkoxysilanes leads to the formation of colloidal three-dimensional polysiloxane particles. Whether these polymeric particles form a stable sol and remain suspended in solution or undergo intermolecular condensation via residual OH groups to form a three-dimensional network (wet gel) will depend on the nature of the sol particles, the solvent, and the polymerization conditions. The stability and properties of the wet gel will depend on the precursor systems and the number of siloxane bonds formed.
Notes & References
C.J. Brinker, Journal of Non-Crystalline Solids, 100, 31-50 (1988)
C.J. Brinker & G.W. Scherer, Sol-gel Science: The Physics and Chemistry of Sol-gel Processing, Academic Press, San Diego 1990
U. Schubert and N. Huesing, Synthesis of Inorganic Materials, 2nd edition. Wiley-VCH, Weinheim 2005
Alain C. Pierre, Introduction to Sol-Gel Processing, Springer Science, New York 1998
F.D. Osterholz and E.R. Pohl, J. of Adh. Sci. and Technol., Vol. 6, pp 127-149 (2012)
H Schmidt, H. Scholze and A. Kaiser, Journal of Non-Crystalline Solids, 63, pp. 1-11 (1984)
-
Water glass solutions are complex mixtures of monomeric and oligomeric silicates such as sodium metasilicate Na2SiO3, sodium orthosilicate Na4SiO4, and sodium pyrosilicate Na6Si2O7.